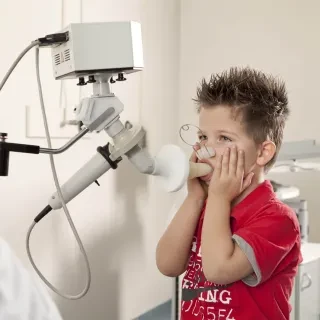
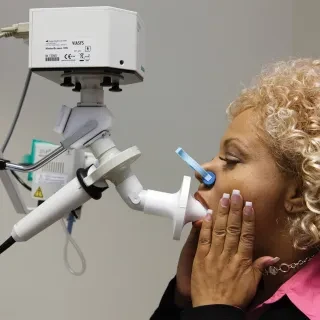
IOS Impulse Oscillometry
Tidal breathing analysis with Vyntus™ IOS (Impulse Oscillometry) has proven to be an informative and meaningful tool used in the early detection and follow-up of pulmonary diseases, including asthma, COPD, and idiopathic pulmonary fibrosis.
IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adults and geriatric patients.
The Vyntus™ IOS allows for the differentiation of central and peripheral airways resistance, whilst also allowing spirometry measurements to be performed.
IOS can be upgraded to the Vyntus™ PNEUMO spirometer as an optional feature, enabling comprehensive pulmonary function testing.
Why Choose Vyntus™ IOS?
- Accurate Lung Function Assessment – Provides precise results with tidal breathing, ideal for patients who struggle with forced maneuvers.
- Early Detection of Airway Dysfunction – Identifies small airway abnormalities even in asymptomatic asthma patients.
- Tracks Bronchodilator Response – Measures treatment effectiveness when FEV1 remains unchanged.
- Seamless Workflow Integration – SentrySuite™ Software offers automatic classification and real-time analysisExpandable Testing Capabilities – Upgrade to the Vyntus™ PNEUMO spirometer for comprehensive pulmonary assessments.
- Vyntus™ IOS is the right choice for precise, patient-friendly lung function testing—empowering clinicians with deeper insights for early diagnosis and effective management.
- Accuracy & Reliability- well-validated and published technology in a wide range of testing populations from small children to adults.
- Easy to Use, Comfortable for Patients- flexible arm allows patients to sit or stand, and software that will guide patients through the test.
- Seamless Workflow & Smart Guidance- assists with test quality and compliance with instant quality feedback according to the latest standards.
- Paediatric Friendly with Incentive Coaching
- Hygienic & Low Maintenance- Disinfect or replace your pneumotach-it’s your choice! When using our MicroGard™ II bacterial/viral filter with each patient, we have validated that your Vyntus™ PNEUMO pneumotach only requires cleaning and disinfecting every 6 months.
2. Based on the Bio Burden DIN EN ISO 11737-1: Report 18AA0193
Please note that all products, services, or features of products and services may not be available in your local area. Please check with your local Jaeger Medical representative. The information provided in this site is intended for healthcare professionals.